What is Silicon
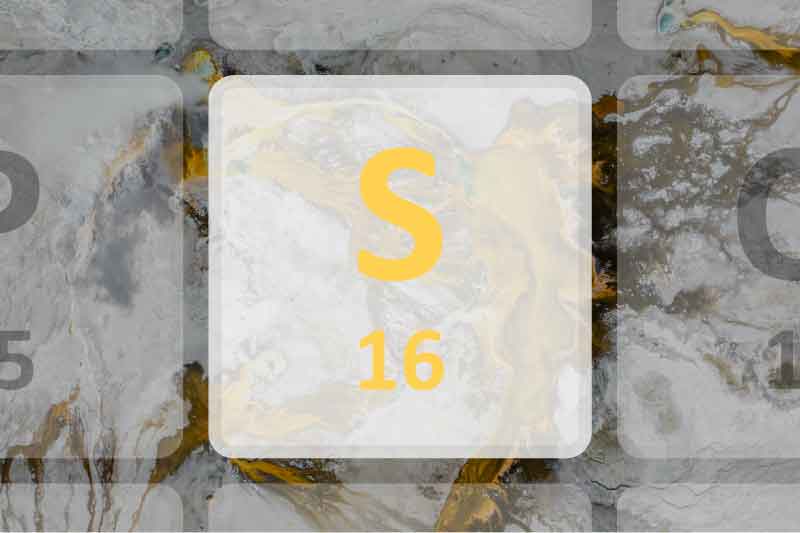
Silicon is an extremely common chemical element. Its chemical symbol is Si. Silicon has an atomic number of 14. it rarely occurs in nature as a single substance. It is often found widely in rocks, gravel, and dust in the form of complex silicates or silica. Silicon is the eighth most abundant in the universe. It is the second most abundant element in the earth's crust.
Silicon has two isotopes: amorphous silicon and crystalline silicon. Crystalline silicon is gray-black and amorphous silicon is black.
The special structure of the silicon atom brings some special properties: higher melting point and density; more stable chemically and difficult to react with other substances at room temperature; no apparent free electrons in silicon crystals. It conducts electricity, but its conductivity is less than that of metals and increases with temperature. This gives it semiconducting properties and is the basis for its use as a raw material for electronic chips.
| I | O | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 H |
II | III | IV | V | VI | VII | 2 He |
||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||
| 3 | 11 Na |
12 Mg |
III | IV | V | VI | VII | VIII | I | II | 13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Gc |
33 As |
34 Se |
35 Br |
36 Kr |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 6 | 55 Cs |
56 Ba |
57-71 La-Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
89-103 Ac-Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Uub |
||||||
| La-Lu | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
|||
| Ac-Lr | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
|||
- Item Name: Silicon
- Element Symbol: Si
- Atomic Number: 14
- Atomic Weight: 28.0855
- Atomic Radius: 117pm
- Density: 2.33g/cm3
- Melting Point: 1410°C
- Boiling Point: 2355°C
- Electronic Layout: [Ne]3s23p2
The Atomic Structure of Silicon
Beneficial Role of Silicon in Superalloys
Antioxidant Properties
Silicon has antioxidant properties in some superalloys such as Incoloy 330. However, silicon is not a good element to add excessively. It has some negative effects on the alloy. Therefore, most alloys rely mainly on chromium to improve their antioxidant properties. Silicon content in the alloys is often limited to less than 1.5%.
330
Si
C
Mn
Cu
Fe
Cr
Ni
Increasing the Durable Life of Low Expansion Alloys
In the low-expansion superalloy Incoloy 909, silicon has been found to significantly increase the durable life of the alloy. As a result, the silicon content of the alloy has been specified at 0.25% to 0.5%. This change significantly addressed the performance issues of previous similar alloys.
909
Si
Mn
Al
Ti
Cu
Co
Nb
Fe
Cr
Ni
Harmful Effects of Silicon in Superalloys
Despite the above two beneficial effects of silicon, silicon is still viewed as a harmful element in most superalloys. Below, we describe three harmful effects of silicon.
Affects Alloy Structure
Silicon in alloys affects the microstructure of the alloy.
First, as an impurity element, silicon is enriched at grain boundaries. This can reduce the strength of grain boundaries.
Secondly, the presence of silicon also leads to the precipitation of harmful phases in the alloy. These harmful phases can lead to cracks.
Finally, silicon also promotes the production of carbides at grain boundaries. This affects the mechanical properties of the alloy.
Reduce Transient Properties
Studies have shown that silicon has a significant effect on the transient properties of alloys. At room temperature, once the silicon content reaches 0.7% or more, the transient properties of the alloy decrease dramatically. This unfavorable effect is mitigated when the temperature is increased to 650°C (which is 1202°F). For some precipitation strengthened alloys, aging treatment amplifies this effect. The effect of silicon on the transient properties is mainly on the tensile strength. For yield strength and hardness, silicon has a limited effect.
Reduce Durable Properties
In addition to transient properties, silicon also affects the durability of alloys. Over extended periods of time, silicon causes cracks to expand in the alloy and increases the likelihood of fracture of the material. This detrimental effect requires the alloy to have less than 1% silicon. Therefore, in summary, silicon in alloys should be kept below 0.7% in most cases.
Conclusion
Silicon is a widely distributed element. In superalloys, silicon can improve the oxidation resistance as well as the durable life of a small number of alloys. However, in most cases, silicon has a detrimental effect on the alloy. It reduces the durability and transient properties of the alloy.
We produce high quality superalloys with strictly controlled silicon content. Please contact us for any of your requirements.