What is Boron
Boron is a non-metallic element. Elemental boron is black or dark brown powder. Its hardness is similar to that of diamond. Boron has high electrical resistance. Its electrical conductivity increases with increasing temperature. Boron becomes a good conductor at high temperatures.
Boron is easily oxidized by air. Diboron trioxide forms a film on the surface, which prevents the boron inside from further oxidation. Boron is insoluble in water. Powdered boron is soluble in boiling nitric and sulfuric acids, and most molten metals such as copper, iron, manganese, aluminum, and calcium.
| I | O | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 H |
II | III | IV | V | VI | VII | 2 He |
||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||
| 3 | 11 Na |
12 Mg |
III | IV | V | VI | VII | VIII | I | II | 13 Al |
14 Si |
15 P |
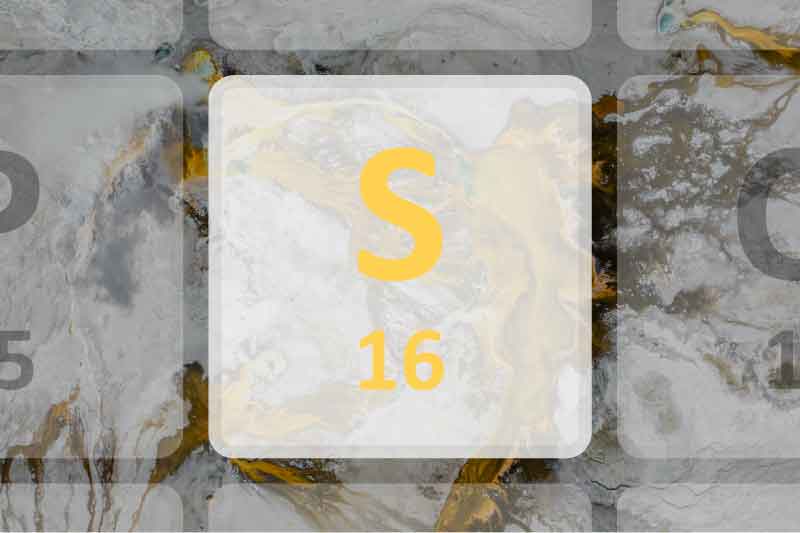
16 S |
17 Cl |
18 Ar |
||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Gc |
33 As |
34 Se |
35 Br |
36 Kr |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 6 | 55 Cs |
56 Ba |
57-71 La-Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
89-103 Ac-Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Uub |
||||||
| La-Lu | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
|||
| Ac-Lr | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
|||
- Item Name: Boron
- Element Symbol: B
- Atomic Number: 5
- Atomic Weight: 10.81
- Atomic Radius: 83pm
- Density: 2.34g/cm3
- Melting Point: 2076°C
- Boiling Point: 3927°C
- Electronic Layout: [He]2s22p1
The Atomic Structure of Boron
Segregation of Boron
One of the most important functions of boron is to improve the durability of the alloy. A small amount of boron will segregate at the grain boundaries of the alloy, increasing the bonding force of the grain boundaries, thereby improving the durability. Studies have shown that the durability of the alloy increases first and then decreases with the increase of boron content. The figure below shows the relationship between alloy durability and boron content:
The Relationship between Alloy Durability and Boron Content
It can be seen from the figure that when the boron content is less, the increase of the boron content will cause the increase of the durability of the alloy. When the boron content is about 0.006%, the durability of the alloy reaches the maximum, which can reach 3~6 times of the initial value. At this time, if the boron content is further increased, the durability of the alloy will drop sharply. Excessive boron content can even have a detrimental effect on the durability of the alloy.
The Role of Boron Compounds
When the boron content reaches a certain amount. It forms compounds with other elements in the alloy. The amount of this boride formed is related to the boron content and temperature. After the boron content reaches 0.004%, borides start to form around 1260°C. When the boron content increases, the boride formation temperature will gradually decrease.
Borides can significantly increase the strength of alloys. Generally speaking, when the temperature of the alloy increases, the strength of the alloy decreases. However, when boron exists in the alloy and the temperature rises to a certain level, the strength of the alloy will rebound due to the formation of borides.
In the actual alloy composition design, we should consider not only the strengthening effect of borides, but also the influence of boron on the durability of the alloy. For some precipitation strengthening superalloys (such as Inconel 740H, Inconel 783 and Incoloy A-286), they will be required to add a small amount of boron. Borides are formed during aging treatment to further increase the strength of these alloys. But their boron content is often specified to be less than 0.012%. This is to avoid the reduction of the durability of the alloy due to excessive boron content.
740H
Si
Mn
Al
Ti
Cu
Co
Mo
Fe
Cr
Ni
783
Si
Al
Ti
Cu
Co
Nb
Fe
Cr
Ni
A-286
Si
C
Mn
Al
Ti
V
Mo
Fe
Cr
Ni
Suppress Harmful Phase
Titanium is present in some alloys. Titanium element and nickel element in superalloy will produce harmful phase Ni3Ti at the grain boundary. This phase is also called the TCP phase. If the TCP phase exists in a large amount, the transient properties and permanent properties of the alloy will be drastically weakened.
Since the boron in the alloy will accumulate at the grain boundaries, they have the effect of inhibiting the precipitation of the TCP phase from the grain boundaries. This increases the stability of the alloy structure.
Improve Casting Performance
In some casting alloys, more than 0.1% boron is added to improve the casting properties of the alloy. The boron in the alloy forms a fine, low-melting liquid during casting. During solidification, these boron-containing liquids solidify before the rest of the alloy. They are finely distributed in the alloy and can play the role of drainage. It makes the alloy more uniform during solidification.
Conclusion
Boron is a non-metallic element. It can improve the durability of the alloy, increase the strength of the alloy and inhibit the precipitation of harmful phases in the alloy. In some alloys, boron can also improve castability.
We can customize alloys with different compositions according to your requirements. If you have any needs, please do not hesitate to contact us.